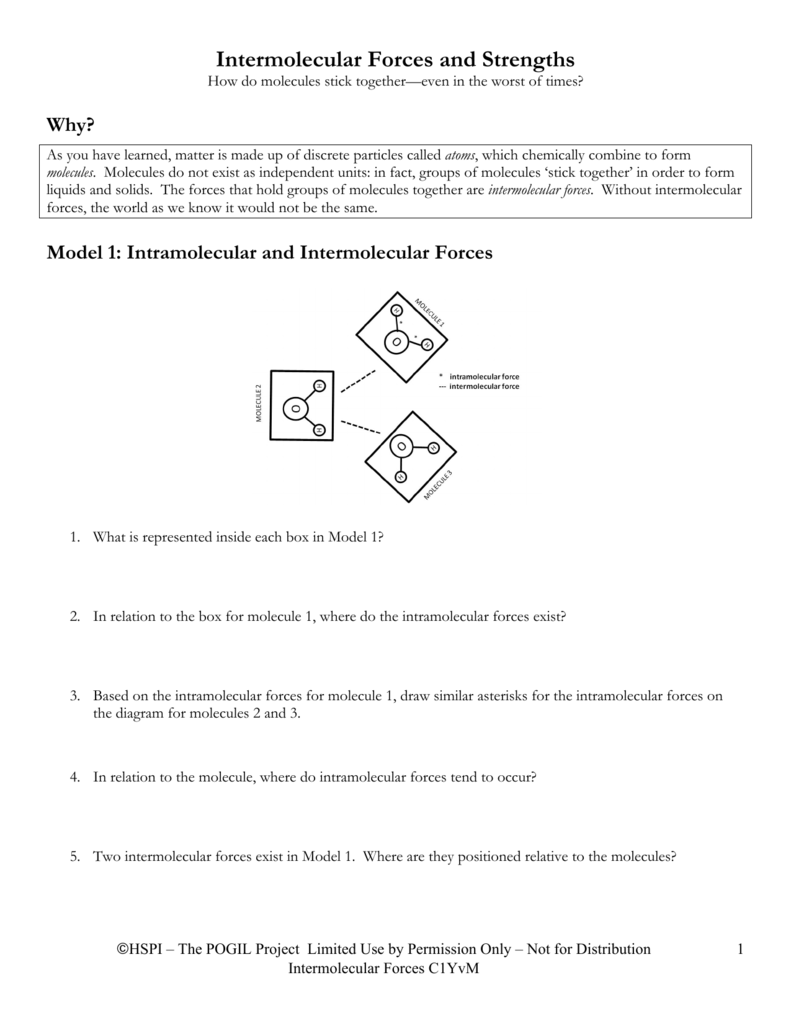
Intro To Intermolecular Forces Pogil Answers | Different types of intermolecular forces (forces between molecules). Intermolecular forces allow us to determine which substances are likely to dissolve in which other substances and what the melting and boiling points of substances are. The intermolecular forces arises due to following interactions: What are these intermolecular forces? In fact, groups of molecules stick. Intermolecular forces allow us to determine which substances are likely to dissolve in which other substances and what the melting and boiling points of substances are. Thank you for submitting your answer. Intramolecular and intermolecular forces critical thinking questions: Intermolecular forces are the forces. Intramolecular forces are the forces within a molecule or between atoms or different parts of a molecule.intermolecular forces are the forces between two molecules.it is normally said that intramolecular forces are stronger because they involve chemical bonding and that is certainly correct. Do the problems on your own before looking at the answers. (a) kr* (b) ncl3** (c) sih4* (d) hf*** (e) n2* (f) nh3*** (g) co** (h) ccl4* 50. Ø intermolecular forces, in addition to being caused by bonding, actually exist within the bonds. Intermolecular forces are the bonds which adjacent molecules form. Intermolecular forceschemistryliquids and intermolecular forceswhat's a liquid?intermolecular forcesthe effects of intermolecular forces the molecules in liquids are held together by forces referred to as intermolecular forces. let's take a look at how they work. Taken from the answer key learn with flashcards, games and more — for free. Greater the intermolecular forces, higher is the boiling point. Determine the kinds of intermolecular forces that are present in each of the following elements or compounds: What is an intermolecular force? Intramolecular forces occur within/inside molecules, while intermolecular forces occur between molecules. Van der waal forces 2. Without intermolecular forces holding molecules together we would not exist. Which of these is not an intermolecular force? intermolecular forces are the attractive forces between molecules the relative magnitude of these forces can also be used to explain trends intermolecular in melting points and boiling points. These forces are weak compared to the intramolecular forces, such as the covalent or ionic bonds between atoms in a molecule. Determine the kinds of intermolecular forces that are present in each of the following elements or compounds: Questions left blank are not counted against you. Worksheet 12a (intro) intermolecular forces predict the type of solids (ionic, molecular, or atomic) the following substances would form: Intermolecular forces are the bonds which adjacent molecules form. What is an intermolecular force? Which of these is not an intermolecular force? Intermolecular forces, which are weaker but hold separate molecules together. Ø intermolecular forces, in addition to being caused by bonding, actually exist within the bonds. Thirty six asked us to determine the kind of in a molecular forces that are present in each element or compound. Do the problems on your own before looking at the answers. Hydrogen bonding van der waals are the weakest van der waal forces exist between all molecules in the universe. Baxley intermolecular forces worksheet answers are on page 3 & 4. (a) kr* (b) ncl3** (c) sih4* (d) hf*** (e) n2* (f) nh3*** (g) co** (h) ccl4* 50. Answer the following to the best of your ability. An intermolecular force is believed. Ø only polar species are involved in intermolecular attractive forces should be drawn between two ends of opposite charge. London dispersion forces are attractive forces that exist between all atoms and molecules. Rank the following from weakest intermolecular forces to strongest. They are a weak electrostatic force and they are caused by the movement of charge. H2se h2s h2po h2te chem128. Thirty six asked us to determine the kind of in a molecular forces that are present in each element or compound. H2se h2s h2po h2te chem128. Ø intermolecular forces, in addition to being caused by bonding, actually exist within the bonds. A chapter eleven sexual problem. (a) kr* (b) ncl3** (c) sih4* (d) hf*** (e) n2* (f) nh3*** (g) co** (h) ccl4* 50. Intermolecular forces are the forces of attraction or repulsion which act between neighboring particles (atoms, molecules, or ions ). Transcribed image text from this question. Taken from the answer key learn with flashcards, games and more — for free. Questions left blank are not counted against you. These forces are weak compared to the intramolecular forces, such as the covalent or ionic bonds between atoms in a molecule. The intermolecular forces arises due to following interactions: Answer the following to the best of your ability. Page 1 of 7 name date block pogil: Which of the following is not a kind of intermolecular force?

Intro To Intermolecular Forces Pogil Answers: These can generally be overcome by physical changes such as temperature.
Refference: Intro To Intermolecular Forces Pogil Answers
0 komentar:
Posting Komentar